Water Beading – What’s the big deal???
So we all know that a waxed surface beads water. If water just lays flat on your paintwork, then it’s time to wax the surface.
But why will a waxed surface bead water in the first place?
Is it naturally occurring because ingredients in the waxes alter the surface energy(low-energy=hydrophobic) and turns it from being hydrophilic to hydrophobic?
Are the ingredients protecting the paintwork(silicones,carnauba, etc.) causing the beading?
Or there are ingredients in the wax dedicated to causing the water to bead?
I try to answer these questions from the perspective of a layman hobbyist detailer. I was curious and searched my university’s journal database on readings about contact angles, wettability, surface protection, etc.
Read on as I try to unravel a detailer's fascination with water beading!
I’m a business undergraduate and was very bad at chemistry in high school(I was having Form 4 & 5 chemistry tuition during my Pre-U days to help me catch up, yes, I was THAT BAD), but I was able to decipher the alien language in some readings that I went through that do suggest that hydrophobicity is related to surface protection.
Firstly, let me define what is wettability and contact angle.
Wikipedia: Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. The degree of wetting (wettability) is determined by a force balance between adhesive and cohesive forces.
Don’t worry, I don’t understand what it means either. What I do know is, water will lay flat on a surface with high wettability, sticking to the topic of this post, an unwaxed surface has high wettability. Conversely, a waxed surface has low wettability as water will tend to bead up and slide off the paint.
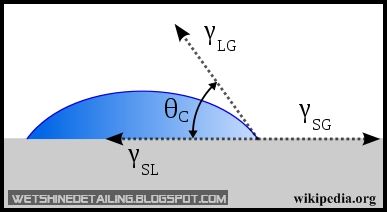
The contact angle on the other hand is the angle at which a liquid/vapor interface meets a solid surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat horizontal solid surface(Wikipedia).
In the image above, θC is the contact angle. Therefore, if the droplet of water has a high contact angle(more than 90 degrees) on the paint, it is said that the paint is hydrophobic.
There are also instruments to measure contact angle, as seen in the video below.
I then try to measure my own contact angle of a water droplet using a photograph and a digital screen protractor. Although it is very crude, it does give me a quantitative value.
The surface in the photograph is a foam applicator that is supplied with Carmate SIV Wheel Coat, as seen in this post.
It may be highly inaccurate, but again, it gives me a rough comparative figure.
So, how does a hydrophobic surface benefit the auto detailing industry? If a wax makes the paint more hydrophobic than the other, does it mean the latter does not protect as well?
This is a question that requires more research. I can’t really find the direct relation between hydrophobicity and surface protection but waxes, sealants and coatings usually cause the surface to be hydrophobic.
For what it’s worth, Emelyanenko(2007) reviewed that low-energy anti-bacterial coatings (that render the surface to be hydrophobic) showed a good resistance to biofouling for a broad set of bacteria cultures.
In addition, Jia and Ling(2005) found in their experiment that steel coated with a hydrophobic polysiloxane coating reduces soil adhesion to the surface. They also commented that soil animals’ skins possess a strong hydrophobic character, therefore, soil animals such as earthworms can freely move in soil and it does not adhere to their skin.
Metals, metal oxides, and inorganic materials are all high surface energy materials and possess hydrophilic character. Conversely, all organic materials are low surface energy materials and have an excellent hydrophobic nature.
I tried to find readings that directly relate wettability to automotive clear coats, there were little results and the ones which I wasn't sure was relevant had to be purchased at about USD34 per article.
It is then well established and well known that waxed paintwork will bead water.
This fact is so well known that contact angle has become a marketing tool.
I remembered my trip to Autobacs in Singapore, where they stocked many Japanese products that were not available in Malaysia.
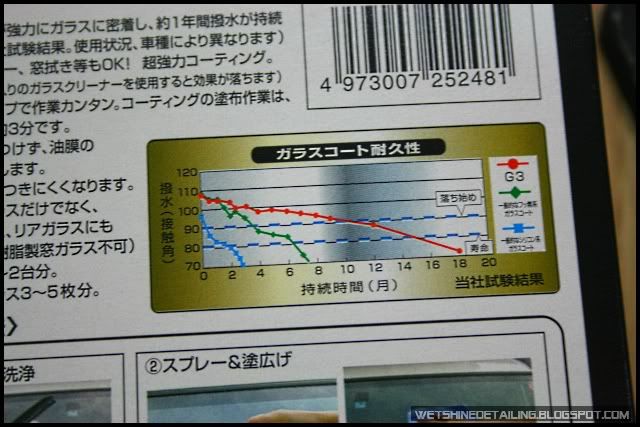
It was interesting to see that on the packaging of almost every Japanese wax/sealant, it had a picture of water beading or a graph showing the contact angle of water over a period of time.
The graph would then point out that the contact angle of their product remains relatively large where as their competitor’s water contact angle would fall dramatically after the first few months.
There was one product which caught my eye. The back of it had beads of water with a fire trail blazing behind them, emphasizing the great speed that water will slide off the paint, LOL.
Compare this with car care products from western countries such as U.S.A and U.K., namely, Meguiar’s and Autoglym. They emphasize greatly on shine and protection their wax will give, and little to no emphasis on water beading at all. (Until recently where Meguiar's implemented their Hydrophobic Polymer Technology in products like NXT 2.0, M21 2.0 Ultimate Quik Detailer and Ultimate Quik Wax)
This can indicate two things. Either the western countries know that hydrophobicity does not benefit the paint in terms of increased protection, or the Japanese, so advanced in their technology, have already found out that hydrophobicity is positively related to surface protection.
Here's why I think water beading is a great marketing tool.
Besides looking at the water beads, how else could you tell the surface is being protected?
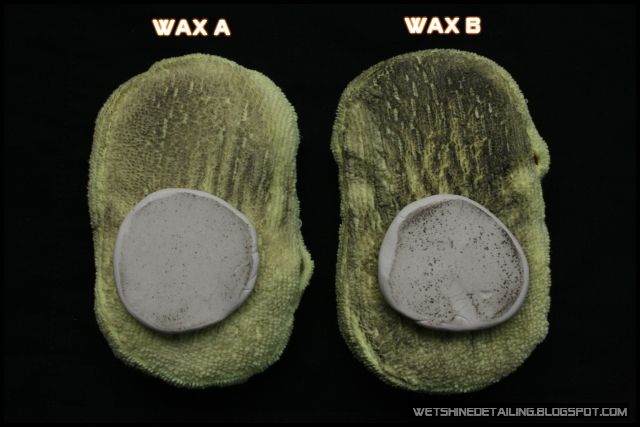
Two ways I can think of, is claying and polishing two different panels and comparing the amount of contaminants accumulated on the clay and applicator. The clay and applicator that shows less contaminants indicates that the wax/sealant/coating being used offers better protection(Wax A in image below).
Now let’s put yourself into the shoes of the salesman trying to sell a car wax. You go to your customer and demonstrate your product, the old burning lighter fluid on the hood trick is old snake oil and doesn’t work anymore.
NOTE: There is NO intention to defame the product demonstrator in the video above!
And you cannot do a clay and polish test since you just applied the product, the only other visual way to assess the performance of the product is hydrophobicity.
If the sealant you are trying to sell pushes water away much faster, create much taller water beads, you will probably close the sale straight away.
Other visual indicators such as gloss and wetness is hard to evaluate quantitatively. Slickness is only comparable for a short period of time since most of the oils are washed away after a few car washes and rain.
Hence, using water beading/contact angle as a visual indicator of product performance makes sense. Using it as a marketing tool makes even greater sense!
I hope you are still following me and see what I’m trying to show you. Your normal traditional wax will turn the surface hydrophobic, companies then use this clue to better market their products by creating more hydrophobic waxes. However,
I wish I was a scientist right now and could do an experiment and give you an answer. Until then, water beading and contact angles would continue being the main selling point of car waxes, sealants and coatings all over the world(refer to Zaino Store).
This is unbeneficial to the society because some companies may exploit this by creating products that are very hydrophobic, but offers little to no protection to the paintwork. I realized this when I did my Soft99 Fusso Coat vs Meguiar’s M21 Synthetic Sealant years ago.
The cost is low and the process is easy in making a surface hydrophobic. There are products out there such as car shampoos that actually creates water repellency. There are spray products that can be sprayed on and the surface will bead water.
But will they actually protect the surface? Possibly, but I assume only to a certain degree. Creating a low surface energy(hydrophobic) is the primary function of those products, actually protecting the paintwork is only secondary(my own assumption).
Let’s look at another example, Aquartz, a new hydrophillic quartz coating from the U.K, which originates from Korea.
Forummer JJ_ from Detailing World posted a review comparing Collinite 476S, G|Techniq C1 and Aquartz. He also posted a video on water behavior on the three different areas of the bonnet.
As you can clearly see in the video above, a high performance quartz coating such as Aquartz does not bead water at all, it has the characteristics of high wettability.
Would you equate this to poor surface protection?
Probably not, due to the hardness of the coating and resistance to environmental attacks. If you compare that surface to an unwaxed surface, I would assume they would look visually similar in terms of water behavior. This is hard to tell and different methods have to be executed to demonstrate the protective capabilities of a quartz coating such as Aquartz.
Caledonia from Detailing World has shown this as seen in his thread, where he heated some high pH chemicals on areas untreated and treated to Aquartz. The treated areas showed resistance to chemical etching and clearly shows strong protective qualities.
It is a very good write-up with strong data to boot, but it would be hard to fit all of that data on the back of a bottle of wax.
Which brings us back to why water beading and contact angle is used as a sensible marketing tool as well as a suggestive indicator of product performance.
See here for example.
All of this marketing makes us love waxes that create tall water beads.
To detailers, having your paintwork bead water taller than the car beside you is akin to a girl walking around with Jimmy Choo shoes next to a girl wearing shoes from Tesco.
However, there are also some who go against the notion of water beading.
When rain falls from the sky, the droplets of water catch and hold atmospheric pollutants on the way down. These dirty rain water droplets land on your car and as they evaporate away, they leave the pollutants and minerals behind, which could etch into your paintwork, forming horrible waterspots that make your car looks like it is going through puberty.
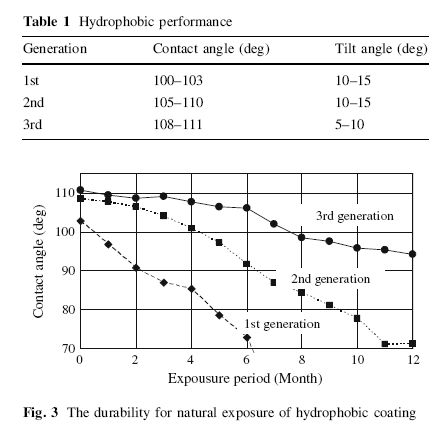
However, findings show that the greater the hydrophobicity of the surface, the greater the tilt angle. The tilt angle, also known as sliding or roll angle, is the angle in which the water droplets slide off the surface.
Hydrophobicity is positively related to tilt angle. So the taller the water beads(larger contact angle) are created, the easier it is for water to slide off the paint.
Then again, what if your car’s horizontal surface is perfectly flat, there’s no use no matter how hydrophobic the surface is, unless the tilt angle is 0, which means water will slide around on a flat surface, like it has a mind of it’s own.
So, hydrophobic or hydrophilic?
To me, I would choose the one that offers greater protection against environmental attacks such as acid rain, tree sap, bird droppings and traffic film. Which means having to test them over a period of time in natural exposures to find the holy grail of paint protectants.
It is not as easy as picking a product that says it is superhydrophobic. Because of this, I am still a cynic of hydrophobic=great protection.
Although I do admit that I would instantly buy a product if it claims that water will roll off from the paint from a small exhale through the nostril.
Additional Info:
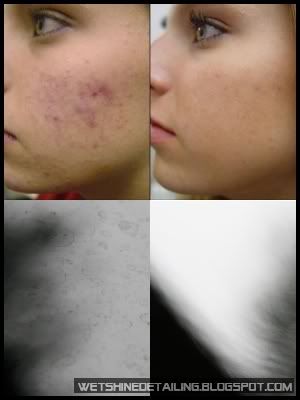
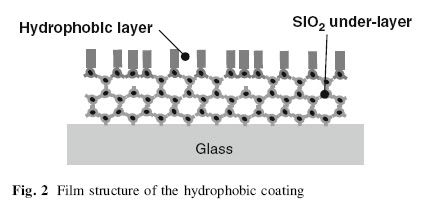
The image above shows the film structure of the hydrophobic coating for Automotive Windows. According to Kamitani and Muromachi(2008) as cited in Aegerter, Almeida, Soutar, Tadanaga, Yang and Watanabe(2008), hydrophobicity is provided by a fluoroalkyl group arranged in good order on film surface. To obtain higher environmental stability and abrasion resistance, a silica under-layer should be applied on the glass surface.
This is just to suggest that hydrophobicity does not always relate positively with the protective qualities of the coating.
All this talk about water beading is making me itch to do my next test, coating test will come soon!
Stay tuned!!! Subscribe via e-mail to receive notifications of new posts.
Subscribe to Wet Shine Detailing by Email
That's all folks, feel free to add your own thoughts on water beading or critique which areas that I am wrong in my post, I would appreciate it!
References:
Emelyanenko, A.M. (2008) Wettability of interface boundaries as an indicator of their properties and state. Protection of Metals, 44(5), 419-429.
DOI 10.1134/S0033173208050019
Jia, X., Ling, X.M. (2005) Reduction of soil resistance through the use of a composite coating. JCT Research, 2(8), 669-672.
Aegerter M.A., Almeida R., Soutar A., Tadanaga K., Yang H., Watanabe T. (2008) Coatings made by sol-gel and chemical nanotechnology. J Sol-Gel Sci Technol, 47, 203–236
DOI 10.1007/s10971-008-1761-9
http://en.wikipedia.org/wiki/Contact_angle
http://en.wikipedia.org/wiki/Wetting
http://www.detailingworld.co.uk/forum/showthread.php?t=168666
http://www.detailingworld.co.uk/forum/showthread.php?t=170993&highlight=aquartz
http://www.markus-bader.de/MB-Ruler/index.htm













18 comments:
so free ah!!!??
LOL
just kidding, good job mate.
HAHAHA! Yes actually quite free for a few days since just finished my final final final paper 2 days ago...
Thanks mate!
so now finished with all exams? great! can look forward to more of these....
anyway, back to the topic; no comments yet, as still trying to remember and understand hydorphobic vs hydroophylic.
Jackson, yup just finished! Take your time with the post, took me a while to understand what I read and wrote too..lol
very nice write up ken.. took a while to read it.. only imagine how long u took to write it..
Nice of you sharing. Come to think about it, shame that I seldom pay attention to water beading. I always feel that the most effective way to dignose wax protection capability is still by claying and inspecting the surface for general defects.
@rayd,
Thanks bro. Haha, I think about 12 hours including the research...
@Dschia,
Nothing shameful man, I agree with you on the claying and inspecting part. Especially waterspotting, our acid rain here is terrible! Wonder how is it in S'pore...
Your thesis on water beading is most interesting Dr. Fish....
Therein lies the question, protection = beading?
Interesting read! Thanks for putting it all together.
Fusso Coar vs Megs #21 was another great test, good thing you also put the link here :)
Ok... I almost conned by another car wash shop in my neighbourhood.
Yes, the guy is somewhat experience or sales trained to show me water beading between Nano wax and Autoglym wax... Of cos, the Nano wax which cost slightly higher bead waters like no other.
Some more he claimed it can sustain 20 washes which I thought it was good buy.
After reading your posting here, it makes sense that water beading may/ may not guarantee on paint protection.
So it goes back to fundamental, how good the paint work from the manufacturer or the repair shop who repainted some panels of your car?
@seech,
Thanks! To summarize the post,
"water beading = suggestive indicator of protection, usually true, but not always."
@Toni,
Thanks, you have a great detailing blog!
@Clement,
Hi Clement, it's been a while, hope you're doing fine. Haha so how much did he wanted to charge you for the wax? You are right, it may or may not be good. Best is to stick to something that is proven, or you frequently use and have delivered results. The paintwork determines how glossy it will look(sanded or not sanded, layers of clearcoat, etc.), the paint hardness determines it's mar resistance. Harder paintwork is more resistance to light scratching such as car wash abrasions and also delays the rate of waterspot etching.
Great writeup indeed. Very entertaining yet satisfying. (Bow Bow)
Fit to be Malaysian Top Blogger!!!
wow Tim....you touch me... (bow 720 degrees)
thanks for the very lengthy discussion. It's really an eyeopener!
Very technical I must say. But hopefully many people now understand why water beading is such a big deal when it comes to auto detailing.
Regular use of car wax will help keep your car looking bright and shiny longer. This is because the wax forms a reflective layer over the paint.
Thanks! I can say that cars inspire feelings of elegance, leadership, and often a sense of wealth that you may not even have.
The value of your car is often affected by how well it is presented. A gleaming vehicle, is clearly worth more.
Post a Comment